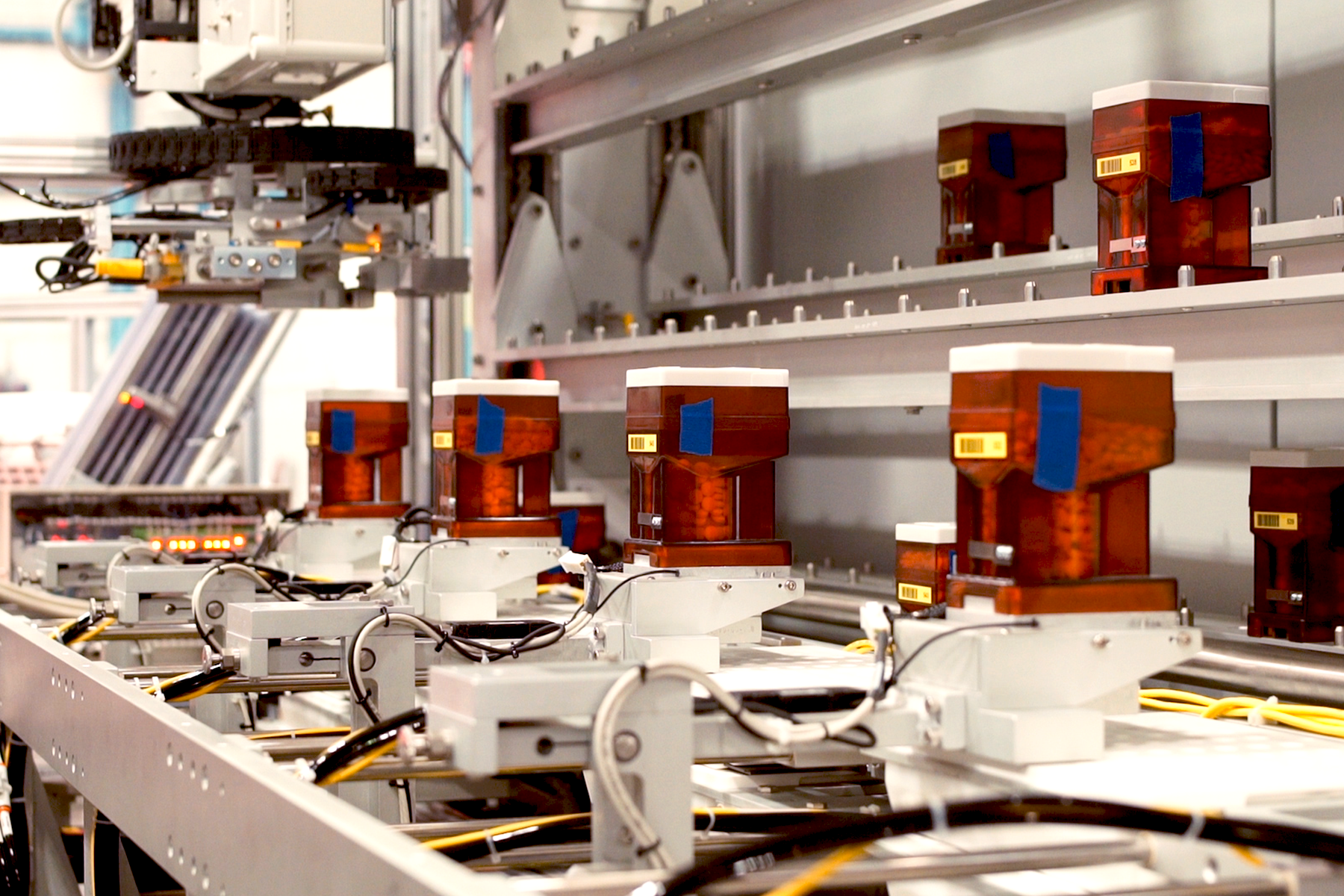
Pharmaceutical Industrial Automation Systems
Engineered for Compliance and Speed
Our automation systems are engineered to help pharmaceutical manufacturers improve efficiency, product quality, and compliance. From material handling to final packaging pharmaceutical products, we deliver pharma solutions tailored to strict demands of regulated environments. Whether you’re scaling up, reducing manual handling, or improving traceability, DT Engineering helps make your facility run smarter and faster.
Our Capabilities
Tablet Press Automation
Capsule Filling & Sealing Systems
Powder Handling & Conveying Systems
Vision Inspection & Reject Systems
Dose Dispensing & Weight Verification
Bottle & Blister Packaging
Track & Trace Systems
Cleanroom-Grade Robotic Handling
Why Automate in Pharmaceutical Manufacturing?
Automation in pharma manufacturing supports consistent quality, reduces safety and contamination risks, and enables traceable, repeatable production. Our pharmacy production systems help reduce cycle times, manual errors, and downtime, delivering better control over complex manufacturing demands while aligning with validation, CFR Part 11 compliance, and audit-readiness requirements.
With automation, pharmaceutical manufacturers gain:
improved traceability
reduced cycle times
scalable batch production
Real-time monitoring and smart analytics empower proactive decision-making, while automated documentation streamlines audits and validations.
Automate with Confidence
At DT Engineering, we provide automation systems purpose-built for the pharmaceutical manufacturing industry. Our solutions are GMP-compliant, CFR 21 Part 11-ready, and designed for cleanroom integration, so your production lines meet regulatory expectations while maintaining high throughput and product purity.
With strategic engineering support, advanced control systems, and turnkey systems, we help manufacturers improve yield, reduce labor reliance, and maintain compliance across every batch.
Key Advantages of Automation for Pharmaceuticals
Automation in pharmaceuticals streamlines production, reduces human error, and ensures consistent product quality. It also enhances regulatory compliance and operational efficiency, making it a vital tool for innovation and competitiveness.
Exceptional Accuracy & Repeatability
Modular & Scalable Systems, Designed For Ease of Validation
Reduced Risk of Human Error & Batch Deviation
Integrated Inspection, Labeling, & Data Logging Aligned with 21 CFR Part 11
21 CFR Part 11 Ready
Digital batch records, e-signature protocols, and secure audit trails.
Real-Time Monitoring and AI Diagnostics
Reduce deviations risks with vision systems and predictive analytics.
Secure Systems Architecture
Cyber-resilient PLC and HMI integration for data integrity and control.
Automated Documentation Suite
Generate validation-ready reports, SOPs, and DQ/IQ/OQ/PQ protocols with minimal manual intervention.
Seamless Validation to Meet FDA Requirements
By partnering with DT Engineering and Sterling Engineering, you’ll reduce your time and resource investment, while capitalizing on our knowledge and expertise in pharmaceutical production equipment. We can provide commissioning assistance of equipment for SAT/FAT and assembly process equipment validation. Our documentation package includes: DQ, SQ, IQ, and OQ. We have deep experience in executing assembly validation processes to help meet FDA requirements.
We provide a complete qualification document package.
Proof of Our Success
Executing Complex Pharma Automation Through Build-to-Print Manufacturing
Designed for blister card medication fulfillment, the system was built to the customer’s exact design specifications, showcasing our capacity to handle complex pharmaceutical manufacturing projects and deliver high-quality, customized solutions.
DT Engineering proved its strength as a reliable and skilled manufacturing partner. The successful execution of this automated engineering project:
Accelerated time-to-market
Improved patient safety
Streamlined regulatory compliance
Reduced cost of ownership